
New Computational Strategy Finds Brain Tumor-Shrinking Molecules
by Zara Jethani
Computer modeling identifies first-ever molecule to inhibit a transient cellular event that drives glioblastoma, and the molecule shrinks glioblastoma in mice.
The optimal treatment for most gliomas is maximal surgical removal followed by radiation therapy and chemotherapy. However, patients with glioblastoma, a type of malignant glioma, unfortunately usually survive less than 15 months following diagnosis. Since there are no effective treatments for this disease, we have created a new computational strategy to search for molecules that could be developed into glioblastoma drugs. In mouse models of human glioblastoma, one molecule we found shrank the average tumor size by half.
The newly discovered molecule works against glioblastoma by wedging itself in the temporary interface between two proteins whose binding is essential for the tumor’s survival and growth. This study is the first to demonstrate successful inhibition of this type of protein, known as a transcription factor.
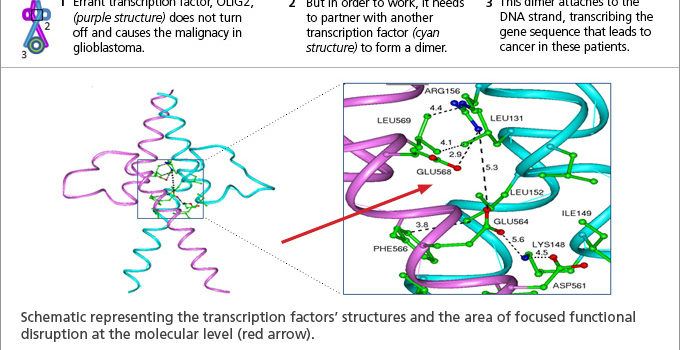
Transcription factors control which genes are turned “on” or “off” at any given time. For most people, transcription factors labor ceaselessly in a highly orchestrated system. In glioblastoma, one misfiring transcription factor called OLIG2 keeps cell growth and survival genes “on” when they shouldn’t be, leading to quick-growing tumors. In order to work, transcription factors must buddy up, with two binding to each other and to the DNA at same time. If any of these associations are disrupted, the transcription factor is inhibited.
In this study, our research team aimed to disrupt the OLIG2 buddy system as a potential treatment for glioblastoma. Based on the known structure of related transcription factors, we developed a computational strategy to search databases of 3D molecular structures for those small molecules that might engage the hotspot between two OLIG2 transcription factors.
With this approach, we identified a few molecules that were tested for their ability to kill glioblastoma tumors. The most effective of these candidate drug molecules, called SKOG102, shrank human glioblastoma tumors grown in mouse models by an average of 50 percent.
While the initial pre-clinical findings are promising, it will be several years before a potential glioblastoma therapy can be tested in humans. SKOG102 must first undergo detailed pharmacodynamic, biophysical and mechanistic studies in order to better understand its efficacy and possible toxicity.
This research was conducted at the UC San Diego, Moores Cancer Center, as well as the San Diego Supercomputer Center and Department of Neurosciences at UC San Diego and appears in the October 30, 2015 issue of Oncotarget.
About the Author

Zara Jethani
Zara is the marketing director at Pacific Neuroscience Institute. Her background is in molecular genetics research and healthcare marketing. In addition, she is a graphic designer with more than 20 years experience in the healthcare, education and entertainment industries.
Last updated: May 3rd, 2022