

Stem Cell Therapy and Brain Health: The Future Is Now
by PNI Experts
Clinical trials using stem cells have shown promising early results as cellular therapies in regenerative medicine, as well as in potential anti-cancer applications.
The use of stem cells as medical treatments is already having an impact on patients in the clinic, according to early results from studies at Pacific Neuroscience Institute (PNI) investigators. The flexibility and adaptability of these cells make them especially exciting new therapeutic options, both for rebuilding damaged brain tissues as well as for combatting cancer.
Exploring Stem Cells as Treatments
Stem cells have the unique ability to continuously replenish themselves, called “self-renewal”, as well as develop into many different types of cells that make up our body (called “differentiation”), such as muscle cells, blood cells, or brain cells.
The goal of stem cells in the body is essentially to maintain homeostasis – which is basically the status quo functioning of our body – by regenerating and replenishing cells over time, such as in the skin or gut. Since they play a key role in injury recovery and healing, PNI experts are using this property of stem cells to boost healing in areas where patients’ own stem cells haven’t been successful enough at their baseline abilities of wound healing.
The goal of the clinical trials program at PNI and Saint John’s Cancer Institute is to address unmet medical needs in our neuroscience patient populations, and support the translation of new therapeutic innovations that improve the quality of lives for our patients.
PNI completed neurosurgical trials to assess the safety and efficacy of stem cells to promote motor recovery in patients with different forms of brain injury, including ischemic stroke and traumatic brain injury (TBI).
The brain is in a protected environment surrounded by what’s called the blood brain barrier, protecting it from exposure to toxins, or immune cells that might cause inflammation and harm. But the blood brain barrier also prevents our immune cells and stem cells from getting in when they need to help fight cancer or help promote recovery of the brain after an injury, like a stroke or concussion.
Using a highly precise, minimally invasive neurosurgical approach, modified stem cells were placed directly into the site of brain injury in stroke and TBI participants. The goal of cellular therapy is to get those stem cells to the areas that they can’t get to naturally, and minimally invasive neurosurgery is a tool for surgeons to deliver these cells exactly where they need to be in the right quantities needed to have an impact.
Both studies were multi-center, international collaborations, with PNI/SJCI leading the overall surgical enrollment worldwide in the ischemic stroke trial. At the time of this writing, Phase 2B stroke study results had not yet been published, but PNI experts were hopeful that the findings will be as positive as earlier Phase 1/2A data that showed the stem cells were safe and associated with significant motor recovery in patients with chronic strokes.
For the TBI study, PNI investigators saw early results that are very encouraging in the recently completed interim analysis. The interim analysis results showed significant improvement of patients treated with stem cells versus the controls, with no safety concerns, which is a significant milestone for regenerative medicine in brain health.
Beyond these particular ischemic stroke and traumatic brain injury trials, the fact that surgeons can deliver stem cells as surgical treatments in the brain with collaborative groups that span the world is really a landmark time in the field, both for clinicians as well as patients.
What’s Next?
Experts hope to see extended indications in other forms of injury to the central nervous system. Examples of this include spinal cord injury, intracerebral hemorrhage (for example when patients have high blood pressure injury to the brain), and neurodegenerative diseases such as ALS and dementia. Using stem cell approaches to boost brain function and prevent effects of the aging brain is of interest in maintaining brain health as well.
Use of Stem Cells in Regenerative Medicine
The implications of regenerative medicine in the future as cellular therapy in neurological diseases are very exciting. Because of the blood brain barrier, the minimally invasive neurosurgical approach is ideal for these clinical trials, as neurosurgeons know exactly where the cellular therapy is being delivered and how much is actually at the target site. While it may be possible to offer effective therapies less invasively in the future, for example by IV, there is more to work out there in terms of how much will actually get to the target site in the brain, and the effects of exposure of other organs to the cells given systemically.
We already have FDA–approved neurodevices like Deep Brain Stimulation (DBS) for Parkinson’s disease where electrodes are placed in specific regions of the brain to increase the activity of cells and promote their function. So surgically placing something biological and not electrical to stimulate cells and promote brain function, is not too conceptually different from existing treatment workflows. Stimulating devices in the brain are similar to devices elsewhere, like cardiac pacemakers, which are electrically telling cells to activate. Newly introduced treatment paradigms with stem cells would be conceptually similar, but relying on a biological tool rather than a device to help promote and boost the function of cells at the site of dysfunction.
For clinical usage in the future, it may become feasible to deliver stem cells less invasively via IV, potentially by genetically engineering the cells so that they are programmed to get to their target site more efficiently.
Autologous vs. Allogenic Stem Cells
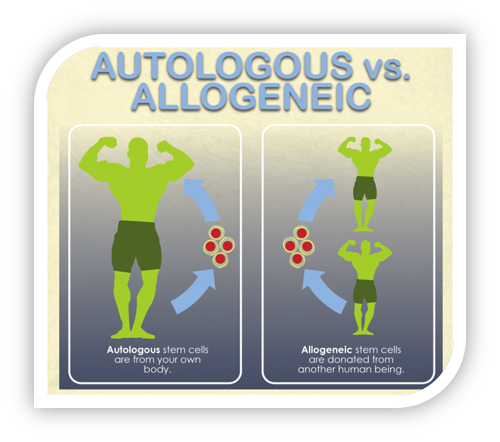
Autologous stem cells are those that are taken from a patient, boosted, and returned to the same patient. Allogenic stem cells are taken from a donor, and conceptually similar to a blood transfusion. There are advantages and disadvantages to each type of cell and studies are being conducted using both. In either case, the stem cells are often modified to help target and boost their activity during treatment and beyond.
The Sanbio stem cell line (used in the stroke and TBI studies) consists of cells that are modified to release trophic and growth factors for wound healing, so that they modulate the immune system to heal rather than being inflamed. The stem cells are linked to the notch-1 protein which specifically helps target healing in neural cells.
The allogenic mesenchymal stem cells used in the Sanbio trials can be viewed as wound healing helpers, like a traffic cop directing signals to promote a healing environment. If they are eventually recognized by the immune system as foreign cells, and are cleared out from the body as a result, they would still have started a process of healing that could have a lasting mechanism of action. In the future, it is possible that allogenic cells from a standardized donation bank can be antigen-matched similar to how it is done in blood banks to limit these immune responses. There is also recent data suggesting genetically-engineering donor cells can prevent immune responses toward them. In allogenic trials the cells presumably initiate healing cascades giving the body’s own cells the capacity for healing with longer lasting effect. The hope is that by limiting or preventing immune clearance of the donor cells there would be an even more pronounced recovery.
Eventually using advanced technologies that are already available, autologous stem cells could be genetically edited so that they don’t ever get cleared from the body but become integrated and help rebuild damaged tissues. It is not too difficult to imagine that these could be used in a similar manner to organ transplants. For example, if the stem cells were brain-specific neural stem cells, those that generate neurons in the brain, they could regrow and rebuild areas of brain tissue left damaged by injury.
Clinical trial exploring Stem Cells for treating Alzheimer’s Disease
PNI experts are currently participating in a phase 2A, multi-center, randomized, single-blind, placebo-controlled, crossover study to assess the safety, tolerability, and preliminary efficacy of a single intravenous (IV) dose of allogeneic human mesenchymal stem cells to subjects with mild to moderate dementia due to Alzheimer’s disease.
Stem Cells as Anti-Cancer Agents
Another aspect of stem cells is their potential as anti-cancer therapies. Scientists can take the stem cell that can regenerate – healing strokes and spinal cord injuries – but rather than using it as a growth promoting agent, it can be used as a factory to create cells in the lab. They can create a line of stem cells that are then differentiated into specific types of immune cells, such as engineered T-cells and anti-cancer myeloid cells, which can be used to specifically seek out and kill cancer cells.
This has relevance as a continuum of leading-edge research at PNI and SJCI. SJCI’s Cellular Therapy Facility has been used for several years to manufacture anti-cancer T-cells to treat patients with melanoma. This resource is now being used to support studies with stem cells in stroke, TBI, and dementia and developing the capacity to combine both these arms of research to create a stem cell-based, personalized medicine workflow for engineering anti-cancer immune cells. In this way, the same Cellular Therapy Facility is supporting treatments not only to help the brain recover from injury, but also to develop anti-cancer immune cells to fight cancers in the brain, like glioblastoma and brain metastases.
A Look Into the Future
Cellular therapies for regenerative medicine and anti-cancer treatments are already reaching patients through advanced clinical trials, and future treatment options are knocking at the door.

An exciting new area of new drug design involves the development of patient-specific cell lines for guiding drug development and treatment selection. One such scenario might include taking a small pinch of skin cells from a patient that could be reprogrammed into stem cells and grown out to create any target cell of interest, such as neural cells in a petri dish.
Diseased neurons in patients with a particular disease like ALS could be compared to healthy neurons on a molecular level. The patient’s disease can be modeled, and drugs created to address the anomalies, all in the dish. Even toxicity studies could be conducted in the lab by testing against cardiac or liver cells made out of the same stem cell bank, ensuring safety before ever initiating a trial in the patient. Such a new paradigm in clinical trial design could help hasten drug development, and could minimize side effects and the type of toxicity events in clinical trials that can otherwise prevent therapies from making it through to the patients who could really benefit from them.
For more information about stem cell clinical trials at Pacific Neuroscience Institute, contact us at 310-829-8265.
About the Author
PNI Experts
Last updated: September 8th, 2022