
Essential Tremor Clinical Trial Opens at Pacific Neuroscience Institute
by Giselle Tamula
Prospective study for symptomatic relief of essential tremor
 January 8, 2019, Santa Monica, CA – The Pacific Movement Disorders Center team is excited to announce that we are one of the sites participating in the PROSPECT trial, a three month study for people who have mild to severe essential tremor.
January 8, 2019, Santa Monica, CA – The Pacific Movement Disorders Center team is excited to announce that we are one of the sites participating in the PROSPECT trial, a three month study for people who have mild to severe essential tremor.
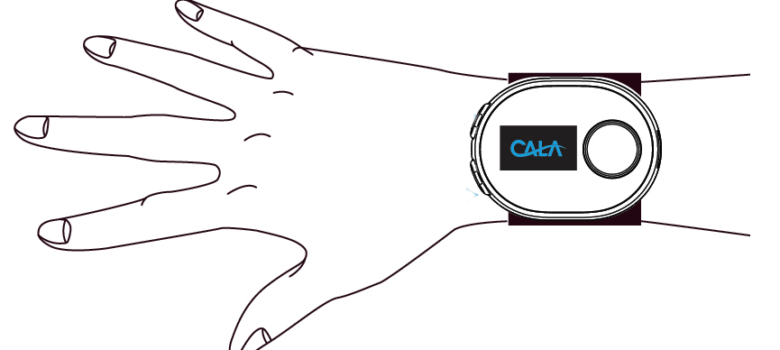
The PROspective study for SymPtomatic relief of Essential tremor with Cala Therapy (PROSPECT) involves the use of a non-invasive investigational medical device, called Cala TWO, at the wrist of the patient’s dominant hand and works by stimulating the radial and median nerves, similar to a TENS unit. The device is about the size of a smart watch and is charged wirelessly.
The device, to be worn twice a day for 40 minutes for three months, will assess any improvement in the tremor, and the tremor reduction duration. This device is very exciting, especially for patients whose tremor is not improved by medication and who are not candidates for, or are interested in, advanced treatment options such as deep brain stimulation (DBS) or focused ultrasound.
What is essential tremor (ET)?
ET is a common neurologic condition affecting over 10 million Americans, and millions more around the world. The “essence” of the disorder is a shaking of a part of the body, more commonly the arms or hands, head, and even sometimes the voice. It can be devastating in its severe form as it makes daily simple tasks more challenging to accomplish.
Activities such as feeding oneself, brushing one’s teeth, buttoning a shirt, carrying a plate, or writing a check may become laborious endeavors. It may be a source of distress or embarrassment to people whose tremor manifests when they speak, not because they have social anxiety, but by virtue of the condition itself. ET can run through families, meaning people with parents that have ET, have a higher risk than the general population of developing the condition. ET is a progressive disease that can increase in severity over a span of decades.
Current Treatment Options
There are currently no pharmacologic therapies that can cure tremor. However, there are a few medications that can help control it. The more common ones used in clinical practice are propranolol and primidone.
Propranolol is a beta blocker that was previously more commonly used to treat high blood pressure, but is now more typically used for ET. It can lower the heart rate; therefore, it cannot be used for people who have low blood pressure or whose heart rate is on the low side. Another medication commonly used to treat ET is primidone. It is an anti-seizure medication, whose exact mechanism of action is unknown, however it acts on the GABA receptors (the neurotransmitter gamma-aminobutyric acid), increasing synaptic inhibition, thereby reducing tremor. It can cause a few side effects that can be difficult for patients to tolerate, such as fatigue, dizziness, and somnolence (sleepiness or drowsiness).
There are other medications that are less commonly used with much less efficacy for tremor control, such as gabapentin and clonazepam. Unfortunately, many patients with tremor either do not feel medications are effective enough, or find the medications stop working after a time, or cannot tolerate the medications.
For those that experience more severe tremors that are bothersome to activities daily living, a surgical intervention, such as DBS, may be warranted. DBS is a neurosurgical intervention wherein an electrode is implanted inside the brain to regulate electrical impulses. This then normalizes the circuitry and function of the brain, resulting in reduction of tremor. While all these interventions are viable treatment for ET, having an in-between treatment option that is non-invasive may prove to be a happy medium for those whose tremor is too severe for medication but not severe enough to warrant an invasive procedure.
Cala TWO Device
The Cala ONE device showed a reduction of the tremor when compared to control sham stimulation in the office setting. As there were only minimal side effects reported in the trial including mild and occasional numbness, tingling and skin irritation, the Cala ONE device was FDA approved in April 2018.
In this next step, investigators will assess the Cala TWO device for duration of effect in participants over the course of the trial. In addition, they will learn whether daily use of peripheral neurostimulation results in the patient experiencing less tremor over time. If this intervention proves to be effective, the Cala TWO device will provide a non-surgical alternative to tremor reduction.
Patients will have three office visits over the three-month study period, at baseline, month 1, and month 3, and be asked to utilize the device for 40 minutes twice a day. This is an open-label study, meaning all participants get the device (there is no placebo or sham group). The device is provided free of cost during the study and insurance will not be billed for these visits. Compensation will be provided for the participant’s time.
We are currently enrolling participants for this newly opened trial. People 22 years old and older with mild to severe tremor, on medication for tremor or not, are invited to participate.
The Cala TWO trial will be available at the following two locations:
- PNI- Santa Monica: 2125 Arizona Avenue, Santa Monica, CA 90404
- PNI- South Bay: 4201 Torrance Blvd., Suite 520, Torrance, CA 90503
For more information and to enroll into the trial, please contact Giselle Tamula, NP, at Giselle.tamula@providence.org.

Giselle Therese U. Tamula, MSN, NP-C, is a certified nurse practitioner with extensive clinical experience. A dedicated caregiver, she brings her competent and compassionate care of patients to the Pacific Movement Disorders Center and Pacific Adult Hydrocephalus Center at Pacific Neuroscience Institute.

Melita Petrossian, MD, is Director of Pacific Movement Disorders Center and is a fellowship-trained neurologist with clinical interests and expertise in movement disorders such as Parkinson’s disease, essential tremor, dystonia, gait disorders, ataxia, myoclonus, blepharospasm, hemifacial spasm, Meige syndrome, spasticity, tics, and Tourette’s syndrome. She also specializes in Parkinson’s-related conditions such as Dementia with Lewy Bodies, progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, primary freezing of gait, and Parkinson’s disease dementia.
About the Author

Giselle Tamula
Giselle Therese U. Tamula, MSN, NP-C, is a certified nurse practitioner with extensive clinical experience. A dedicated caregiver, she brings her competent and compassionate care of patients to the Pacific Movement Disorders Center at Pacific Neuroscience Institute.
Last updated: March 10th, 2021